Pressure-Driven Membrane Filtration Processes
Membrane filtration is a method of separating particles in liquid solutions or gas mixtures. This technique is used in a wide range of applications ranging from dairy processing to wastewater treatment. The semi-permeable membrane acts as a barrier which retains larger particles, while allowing smaller molecules to pass through the membrane into the permeate.Molecules naturally move from areas of high concentration to low concentration. By applying external pressure, molecules can then flow from areas of low concentration to high concentration. The pressure difference on both sides of the membrane will cause the permeate to cross the membrane at a steady state. This allows the final product, permeate or retentate to be have higher overall yields.
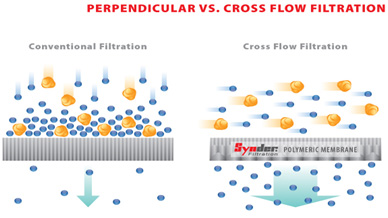
There are several advantages to using cross-flow membrane filtration process:
- Lower energy usage which in turn can cut down the operating cost
- Fewer chemical additives required to remove impurities (e.g. flocculants for wastewater treatment)
- Improvement in production efficiency and quality control
Types of Filtration
The four main methods of pressure-driven membrane filtration are Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis, in order of decreasing pore size.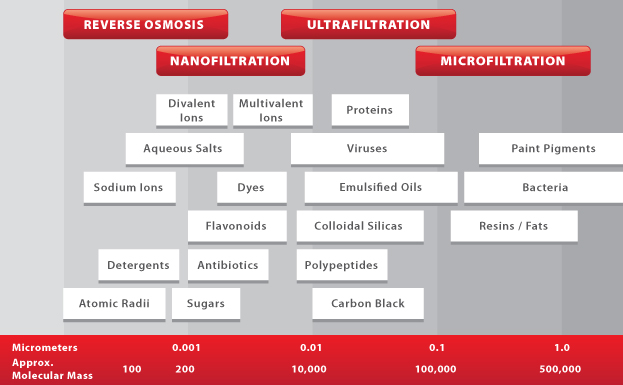
Microfiltration (MF)
Microfiltration membranes have pore sizes ranging from 0.1 to 10 µm. They are mainly used for the removal of large particulates, colloids, and bacteria from feed streams. This is especially popular in the food & beverage industry for treating wastewater before discharging it to a municipal sewer.Ultrafiltration (UF)
Ultrafiltration is a process that is similar to microfiltration, but with smaller pore sizes ranging from 0.01 to 0.1 µm. UF membranes are used in rejecting viruses and polypeptides, and are widely used in protein concentration and wastewater treatment.Nanofiltration (NF)
Nanofiltration membranes are similar to reverse osmosis membranes in that they contain a thin-film composite layer (<1 μm) on top of a porous layer (50 to 150μm) for small ion selectivity. NF membranes are able to reject multivalent salts and uncharged solutes, while allowing some monovalent salts to pass through. They can also operate at lower pressures than reverse osmosis membranes, making them ideal for achieving an optimal combination of flux and rejection.Reverse Osmosis (RO)
Reverse osmosis membranes are even tighter than nanofiltration membranes, and are able to reject all monovalent ions while allowing water molecules to pass through in aqueous solutions. They can also remove viruses and bacteria found in feed solutions. Common applications for reverse osmosis filtration include seawater desalination and industrial water treatment. It is important to note that since the operating pressure for RO and NF is much higher than the pressure applied by MF and UF, the overall yield is relatively lower than that of MF and UF membranes.Membrane Compaction
Membrane compaction is a phenomenon that occurs in pressure driven membrane processes, where pressure tests the mechanical strength of the polymeric membrane The upper pressure limit for Synder’s ultrafiltration and microfiltration membranes are 120 PSI, while the upper limit for nanofiltration membranes are 600 PSI. Once the membrane has been compacted to a certain level, the permeate flux begins to stabilize and fluctuate less. The flux response to pressure during membrane compaction is illustrated in the drawing below. The deformation caused by membrane compaction is often irreversible, especially for flat sheet membranes. If no membrane compaction occurred, flux would have a linear correlation with pressure. In addition, the compaction rate is also proportional to increases in both pressure and temperature. Compaction occurs more frequently in reverse osmosis since the applied pressures are relatively high. In some applications, operating pressures may reach upwards of 1000 PSI. Reverse osmosis and nanofiltration membranes have much smaller pore sizes compared to ultrafiltration and nanofiltration membranes, and therefore require operation at much higher pressures. However, compaction may also occur in ultrafiltration and microfiltration processes, depending on the pressure employed.CASE STUDY
Applications
Resources
MEMBRANE RESOURCES
- Definition of a Membrane
- Membrane Materials: Organic vs. Inorganic
- Pressure-Driven Membrane Filtration Processes
- Concentration Polarization in Pressure-Driven Processes
- Degrees of Membrane Separation
- Flux Behavior in Membrane Processes
Module Configurations & Processes
-> View all membrane resourcesTUTORIALS









